1. Electron that wiggling around nucleus will give off light and Electromagnetic radiation.
2. Every atom will emits different kind of light. Each type of atom gives off a unique set of colors. The colored lines (or Spectral Lines) are a kind of "signature" for the atoms.It's kind of like wearing our team color .Example,look figure 1 :
2. Every atom will emits different kind of light. Each type of atom gives off a unique set of colors. The colored lines (or Spectral Lines) are a kind of "signature" for the atoms.It's kind of like wearing our team color .Example,look figure 1 :
Figure 1
3. If we put light from a common streetlamp through a prism, or look at the light through a diffraction grating, we will see distinct lines. Two common kinds of street lights use sodium vapor and mercury vapor bulbs. Each of these lights has a different spectral "signature", and we can tell what kind of lamp it is by its spectral lines.See figure 2 :
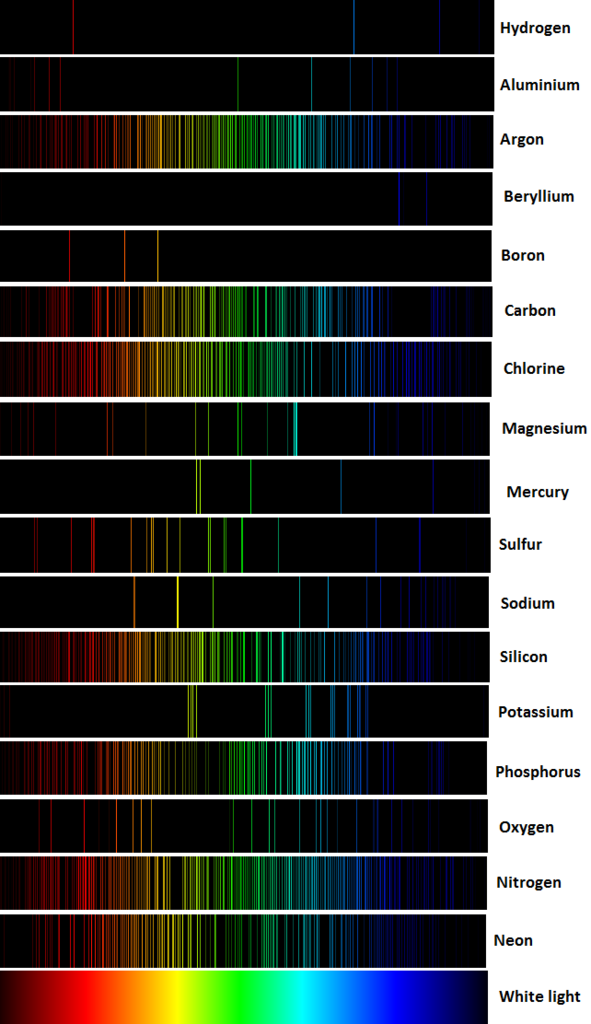
Figure 2
4. From the spectral lines,scientists can tell what elements they are looking at just by reading the lines.
5. Spectroscopy is the science of using spectral lines to figure out what something is made of. That's how we know the composition of distant stars, for instance.





0 comments:
Post a Comment